How Many Ions In Nacl
i.6: Ions
- Page ID
- 46530

Figure 1-5 Common table common salt (sodium chloride. NaCl) is built from closely packed sodium ions, Na+ (modest spheres). and chloride ions. CI- (large. colored spheres). Each ion of 1 charge is surrounded by six ions of the reverse charge at the four compass points and in a higher place and below. This is a particularly stable arrangement of charges. and information technology occurs in many salts. From Dickerson and Geis. Chemistry, Matter. and the Universe The Benjamin / Cummings Publishing Co .. Menlo Park. Ca .. © 1976 .
The idea of a covalent bond suggests equal sharing of the electron pair by the bonded atoms, but the brief discussion of polarity in Department ane-4 indicated that the sharing is non always equal. The relative electronegativity or electron-attracting power of atoms is of great importance in explaining chemical behavior, and is treated in detail in Capacity nine and 10. Sodium atoms (and all metals in full general) take a weak concur on electrons, whereas chlorine atoms are very electronegative. Hence in common table table salt (sodium chloride, NaCl), each sodium atom, Na, loses one electron (e -) to form a sodium ion, Na+. Each chlorine atom picks up one electron to get a chloride ion, Cl-:
-
- Na → Na+ + e - give-and-takeandword
 Cl 2 + e - → Cl-
Cl 2 + e - → Cl-
- Na → Na+ + e - give-and-takeandword
Nosotros write  Cl2 because free chlorine gas exists as diatomic (ii-atom) molecules, non equally free chlorine atoms. Solid sodium chloride (Figure 1-5) has sodium and chloride ions packed into a three-dimensional lattice in such a way that each positive Na+ ion is surrounded on four sides and top and bottom by negative Cl- ions, and each Cl- is similarly surrounded by six nearest neighbor Na+ ions. This is a particularly stable arrangement of positive and negative charges.
Cl2 because free chlorine gas exists as diatomic (ii-atom) molecules, non equally free chlorine atoms. Solid sodium chloride (Figure 1-5) has sodium and chloride ions packed into a three-dimensional lattice in such a way that each positive Na+ ion is surrounded on four sides and top and bottom by negative Cl- ions, and each Cl- is similarly surrounded by six nearest neighbor Na+ ions. This is a particularly stable arrangement of positive and negative charges.
Metals in general lose one to three electrons easily to become positively charged ions, or cations:
-
-
Li → Li+ + east - ttt lithium ion Na → Na+ + east - ttt sodium ion K → K+ + e - ttt potassium ion Mg → Mgii+ + 2e - ttt magnesium ion Ca → Ca2+ + 2e - ttt calcium ion Al → Althree+ + iiieast - ttt aluminum ion
-
Some nonmetals, in contrast, pick up electrons to become negatively charged ions, or anions:
-
-
 F2
F2 + eastward - → F- ttt fluoride ion  Clii
Clii + e - → Cl- ttt chloride ion  O2
O2 + twoeast - → Otwo- ttt oxide ion  Southwardii
Southwardii + 2e - → S2- ttt sulfide ion
-
-
-
-
-
-
-
- Table 1-4 Some Elementary Ions of Elements
-
-
-
-
-

Other simple ions made from single atoms are shown in Table 1-4. The charge on a simple, single-cantlet ion such as AP+ or S2- is its oxidation country or oxidation number. Information technology is the number of electrons that must be added to reduce (or removed to oxidize) the ion to the neutral species:
-
- Reduction: AIiii+ + 3 e- → Al
- Oxidation: S2- → Due south + 2 e-
Pulling electrons away from an cantlet or removing them altogether is oxidation. Adding electrons to an cantlet or merely shifting them toward it is reduction.
| Is chlorine oxidized or reduced in forming the chloride ion? What is the oxidation state of the ion? |
| Solution Chlorine is reduced, since one electron per chlorine atom is added to form the ion. The chloride ion, Cl- , is in the - 1 oxidation country. |
| When metals are converted into their ions, are they oxidized or reduced? What is the oxidation state of the aluminum ion? |
| Solution Metals are oxidized to their ions, since electrons are removed. The aluminum ion, AP+, is in the +3 oxidation state. |
If 2 or more oxidation states for a metallic ion are possible, they are differentiated by writing the oxidation country in Roman numerals later the name of the cantlet. An older classification, yet in use, identifies the higher oxidation state by the ending -ic and the lower by -ous. Hence,
-
-
Fe2+ tt atomic number 26(Two) or ferrous break Atomic number 263+ tt iron(III) or ferric Cu+ tt copper(I) or cuprous break Cu2+ tt copper(Two) or cupric Sn2+ tt tin(Ii) or stannous break Sn4+ tt tin(4) or stannic
-
| When the ferric ion is converted to the ferrous ion, is this an oxidation or reduction? Write the equation for the procedure. |
| Solution The equation is Iron3+ + eastward - → Fe2+ . The procedure is a reduction since an electron is added. |
The modern nomenclature with Roman numerals is easier to use considering it does not require you lot to remember what the ii oxidation states of a metal are, in order to know what a compound is from its name.
A salt is a compound made up of positive and negative ions. Because a salt must be electrically neutral, the full charge on its positive and negative ions must be zero. Since each ion of Sn2+ has a charge of +2, twice as many chloride ions with -1 charge each are required to produce a nothing net charge. Hence the salt of Sn2+ and Cl- ions has the overall composition SnCl2, rather than SnCl or SnCl3. Information technology is chosen stannous chloride or tin (Ii) chloride. The formula for stannic chloride or tin(4) chloride is SnClfour.
In improver to these simple ions, compound or complex ions tin be formed between a metallic or nonmetal and oxygen, chlorine, ammonia (NH3), the hydroxide ion (OH-), or other chemical groups. The sulfate ion, SO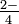 , has four oxygens at the corners of a tetrahedron around the cardinal sulfur atom, and an overall accuse of -2. The nitrate ion, NO
, has four oxygens at the corners of a tetrahedron around the cardinal sulfur atom, and an overall accuse of -2. The nitrate ion, NO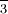 , has three oxygen atoms in an equilateral triangle around the nitrogen, and a -i charge. The ammonium ion, NH
, has three oxygen atoms in an equilateral triangle around the nitrogen, and a -i charge. The ammonium ion, NH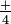 , has four hydrogens at the corners of a tetrahedron, and a +1 charge. These ions are idea of as units because they grade salts the way single-atom ions do, and go through many chemic reactions unchanged. Silver nitrate, AgN0three, is a salt containing equal numbers of Ag+ and NO
, has four hydrogens at the corners of a tetrahedron, and a +1 charge. These ions are idea of as units because they grade salts the way single-atom ions do, and go through many chemic reactions unchanged. Silver nitrate, AgN0three, is a salt containing equal numbers of Ag+ and NO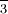 ions. Ammonium sulfate is a salt with twice as many ammonium ions, NH
ions. Ammonium sulfate is a salt with twice as many ammonium ions, NH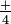 , as sulfate ions, SO
, as sulfate ions, SO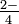 , and the chemical formula (NH4)twoS0four. Other typical circuitous ions are shown in Table 1-5.
, and the chemical formula (NH4)twoS0four. Other typical circuitous ions are shown in Table 1-5.
-
-
-
-
-
-
- Table 1-5 Some Common Complex Ions
-
-
-
-
-

When a primal atom is surrounded by several equally spaced atoms, the number of surrounding atoms is called the coordination number. The most of import cistron is size. Nitrogen in the nitrate ion, NO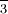 , has room for three oxygen atoms around it, and hence a coordination number of 3 for oxygen. The sulfur atom is larger than a nitrogen atom, and can accommodate ane more oxygen atom in the sulfate ion, SO
, has room for three oxygen atoms around it, and hence a coordination number of 3 for oxygen. The sulfur atom is larger than a nitrogen atom, and can accommodate ane more oxygen atom in the sulfate ion, SO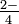 . Hence the coordination number of sulfur for oxygen is 4.
. Hence the coordination number of sulfur for oxygen is 4.
The well-nigh common coordination numbers are 2, 3, four, and 6, (Encounter Table 1-6.) An ion or molecule with a primal atom having a coordination number of 2 can exist either linear, as carbon dioxide with O-C-O in a straight line, or bent, as in water, Htwo0. Possible structures for ions or molecules with coordination numbers of 3, 4, and 6 are shown in Tabular array 1-6.
-
-
-
-
- Table i-half dozen Common Coordination Numbers
-
-
-


Effigy 1-half-dozen Geometry of atoms effectually key atoms with coordination numbers iii, four, and 6. If L is whatsoever peripheral cantlet and M is the central cantlet, and then the bond angle L - M - Fifty is 120° for trigonal planar, 109.v° for tetrahedral, and typically around 109.5° for trigonal pyramidal geometries. Square planar and octahedral geometries take two L - Grand - 50 angles, ninety° and 180°.
It is not strictly correct to talk almost molecular formulas and molecular weights of salts, since there are no molecules in salts-only ordered lattices of ions. No one sodium ion in the sodium chloride structure shown in Figure 1-5 "belongs" to a particular chloride ion. Information technology is correct, however, to speak of the chemical formula of a table salt, and the formula weight that corresponds to it. Since the chemical formula for sodium chloride is NaCl, the formula weight of sodium chloride is the sum of the atomic weights of one atom of sodium and one atom of chlorine:
-
-
i sodium: tt 22.990 amu i chlorine: tt 35.453 amu Total: tt 58.443 amu
-
Information technology is conventional to call this the "molecular weight" of sodium chloride, and no defoliation results as long as you lot realize what a salt construction is like. A mole of sodium chloride is 58.443 g. Information technology will comprise 6.022 10 1023 sodium ions and 6.022 Ten 1023 chloride ions. Even though they are non paired off into molecules, the ratio is strictly one to one.
| What is the molecular weight of ammonium sulfate? | |||||||||||||||||||||||||
| Solution The chemical formula of ammonium sulfate is (NHfour)2And soiv, so the molecular weight (actually the formula weight) is
|
The simple anions are named by adding -ide to the name of the element, as in the fluoride (F-), chloride (Cl-), oxide (Otwo-), and sulfide (Due southtwo-) ions. Where more than than ane complex anion of an element with oxygen tin be formed, the suffixes -ate and -ite are used for the college and lower oxidation states, respectively. Thus,
-
-
Sulfate ion: tt Then 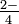
word Sulfite ion: tt And then 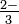
Nitrate ion: tt NO 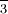
word Nitrite ion: tt NO 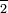
Arsenate ion: tt AsO 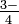
word Arsenite ion: tt AsO 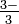
-
If more than 2 such anions exist, then the prefixes hypo- ("under") and per- ("beyond") are used:
-
-
Perchlorate ion: tt ClO 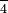
Chlorate ion: tt ClO 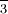
Chlorite ion: tt ClO 
Hypochlorite ion: tt ClO 
-
Melting Points and Humid Points of Salts
A common salt crystal represents a particularly stable balance of positive and negative charges, with each type of ion existence kept out of the fashion of others of like charge. Melting a salt crystal means upsetting this delicate balance of charges, and allowing mutually repelling ions to come closer together from time to time as the ions menstruation past one another. This disruption of structure requires big amounts of free energy to accomplish, so the melting points of salts are higher than those of molecular solids. The melting points of ii salts, sodium chloride (NaCI) and potassium sulfate (K2Theniv), are compared in Table 1-seven with those of the elements from which the salts are made.
| Substance | Chemical Formula | Tm(°C) | Tb(°C) |
|---|---|---|---|
| Sodium metal | Na | 97.8 | 882.9 |
| Chlorine Gas | Cl2 | -101.0 | -34.six |
| Sodium Chloride (salt) | NaCl | 801 | 1413 |
| Potassium metallic | K | 64 | 774 |
| Sulfur | South | 119 | 445 |
| Oxygen gas | Oii | -218 | -183 |
| Potassium sulfate (salt) | GrandiiThen4 | 1069 | 1689 |
Metal sodium melts at 97.8°C, and solid chlorine melts at -101°C, but their combination, sodium chloride (mutual table common salt), requires a temperature of 801°C before information technology will melt. Boiling or vaporizing a salt is fifty-fifty more difficult. The ions remain ions in the liquid land, tumbling by i another as in whatever other liquid; but before the gas phase tin can be attained, Na+ and CI- ions must pair off into neutral NaCl molecules. To achieve this pairing, electrons take to be pulled away from CI- ions, which accept a strong allure for them, and pushed toward Na+ ions, which do not want them. The NaCl bond in sodium chloride vapor is extremely polar, with the electron pair skewed strongly toward the chlorine atom, but the separation all the same is not as complete as in Na+ and CI- ions. Much free energy is required to push electrons where they are not wanted and to make NaCl molecules from Na+ and CI- ions, so loftier temperatures are required earlier this tin can happen. Hence the very high boiling points of salts in comparing with molecular compounds, as illustrated in Table 1-7.
Contributors and Attributions
-
R. E. Dickerson, H. B. Grey, and Chiliad. P. Haight, Jr. Content was used from "Chemic Principles", an introductory college-level text for Full general Chemical science with permissionof the Caltech library and Harry B. Gray, on behalf of the authors.
How Many Ions In Nacl,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book:_Chemical_Principles_%28Dickerson%29/01:_Atoms_Molecules_and_Ions/1.6:_Ions
Posted by: mooremushmers.blogspot.com


0 Response to "How Many Ions In Nacl"
Post a Comment